Is recycled PET safe for contact with food?
Is recycled PET safe for contact with food?
| Author: Patrick Semadeni
In December, the European Commission presented a successor regulation to the existing Regulation (EC) 282/2008 on materials and articles made from recycled plastic that is intended to come into contact with food. Under the previous regulation, not one single recycling process was approved by the Commission, none even for PET. Why is that? How safe is recycled PET for contact with food?
Recycled PET has been in our everyday life for years
Bottles of mineral water and other beverages made from recycled PET, for example, have been in use for years. In the absence of European approval, approval is the responsibility of the each country’s national authorities. The European Food Safety Authority EFSA has classified over 200 recycling processes for plastics as safe, most of them for PET. Despite this, no formal approval has yet been granted by the Commission, as required by Regulation (EC) 282/2008.
How the EFSA assesses the safety of recycling processes for PET
In 2011, the EFSA published the process and criteria for the safety assessment of PET bottles.1 This assessment is based on three pillars:
- Concentration of Post Consumer Recycling (PCR) substances:
The EFSA assumes that the amount of contamination with PCR substances in the washed flakes (after sorting, the bottles delivered for recycling are shredded into flakes, washed and dried) is 3 mg/Kg.2
- Cleaning efficiency of the recycling process;
The crux of the safety assessment is to determine the effective cleaning efficiency of the recycling process.
To do this, the flakes are contaminated with so-called surrogates, which are supposed to simulate PCR substances. Such surrogates are, for example, toluene, chlorobenzene, benzophenone or methyl stearate. The surrogates are supposed to represent different molar masses and polarities as can occur with PCR substances.
These flakes then undergo the cleaning process and the reduction in contamination with the surrogates is expressed as a percentage. The following is an illustration from an EFSA Safety Assessment:3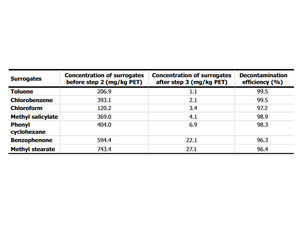
- Consumer exposure to any PCR substances.
Using modeling the EFSA determines for the surrogates the permissible amount of the substance that may still be present in the PET flakes after the cleaning process without posing a risk to consumers.
The modeled allowable surrogate contamination in the PET flakes (Cmod) is then compared to the calculated concentration after the recycling process (Cres) based on the efficiency of the cleaning process.
The residual concentration of a surrogate in the PET flakes after the cleaning process must be lower than the calculated modeled allowable concentration. The recycling process is then considered safe. Here is an example of a safe process4: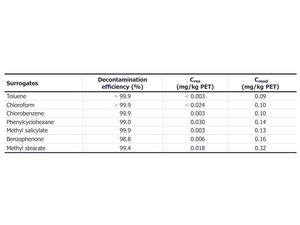
The EFSA considers PCR substances to be potentially genotoxic according to the precautionary principle. According to the TTC (Threshold of Toxicological Concern) concept such substances have a maximum intake limit of 0.0025 µg/kg of body weight per day5.
In the underlying exposure scenario, the EFSA focuses on the most vulnerable group, small children6, unless there is a restriction on the use of a certain foodstuff that it must not be given to children. The corresponding assumptions are included in the modeling of the maximum allowable concentration of surrogates in PET flakes after the cleaning process.
Is the EFSA too conservative in its assessment?
The scientists Roland Franz and Frank Welle are critical of the EFSA's methodology in a recently published paper7. This is also taking into consideration that around 20% of plastic is used for food packaging, and recycling it is indispensable to achieving a circular economy.
Specifically, Franz and Welle state the following:
- As described above, the EFSA assumes that PET flakes for recycling are contaminated with 3 mg/Kg of PCR substances. These values come from a study made more than 20 years ago. Franz and Welle assume that the number of incorrectly used PET beverage bottles has decreased due to changes in the market and that, as a result, the initial contamination of the flakes must be lower.
- The assumption that all PCR substances have a genotoxic effect must be called into question. The EFSA itself writes in its Scientific Opinion of 20111 that genotoxic substances must not be used for consumer goods in the EU and that contamination of PET bottles with them will therefore only occur sporadically, if at all. And even if there are structures present in substances that could possibly have a genotoxic effect, these would react in the recycling process as a result of thermal stress, which, according to EFSA, would further reduce their concentration. In any case, Franz and Welle have not found any publication that proves the presence of genotoxic substances in PET flakes above the detection limit of 0.1 mg/Kg for volatile or 0.5 mg/Kg for non-volatile substances.8
- According to Franz and Welle, the exposure scenarios are unrealistic. In the case of mineral water, for example, the EFSA assumes that the entire quantity consumed per day is taken from 100% recycled bottles, which have also been in storage for a year.
- After all, the EFSA uses models for the calculation of migration, which show values that are too high, even though there are more realistic models. The EFSA is aware of this and corrects the migration values for PET recycling processes by a factor of five. However, this correction is applied across the board to all substances, regardless of whether they have a high or low molecular weight. Franz and Welle assume that for a molecular weight of 300 g*mol-1 the model currently used by the EFSA overestimates migration by a factor of 350. The graphic below compares the calculated migration according to the model currently used by the EFSA (“Piringer") and Welle’s model:9
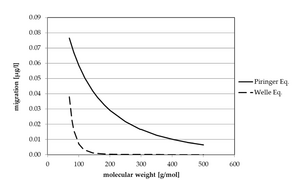
- In an earlier study9 Franz and Welle only found substances in PET that have a molar mass of approx. 200 g*mol-1. Accordingly, larger molecules can either barely or not at all penetrate the PET matrix. In this context, the question arises whether the use of methyl stearate with a molar mass of 298.51 g*mol-1 is realistic. Further, the EFSA also makes no distinction in the initial contamination with regard to the molar mass of the surrogates, despite the different diffusivity. Thus, the initial contamination with the volatile toluene with a molar mass of 92.14 g*mol-1 is set at the same level as higher molecular weight surrogates, even though there are differences in the ability to penetrate into the PET, namely always at 3 mg/Kg.
Conclusion: Reconciling the precautionary principle with the risk approach
We cannot avoid dealing with the question of acceptable residual risk, even if this is something we would like to avoid. With a purely hazard-based approach (pollutant=potential hazard), it will barely be possible to achieve a circular economy, not just for plastics, but for other materials too.
So that leaves a risk-based approach: Pollutant+exposure = hazard. Here the aim is to reflect reality as best we can and to provide a sufficient safety margin to satisfy the precautionary principle.
The more we know about the presence of PCR substances and their toxicological potentials, the closer we come to this goal and the more precisely we can define the required safety margin.
The EFSA's current methodology appears to provide a very high level of safety by assuming worst-case scenarios for concentration, genotoxicity and exposure. The assumptions therefore provide very high margins of safety.
It can thus be assumed that recycling systems for PET, which are classified as safe by the EFSA, are also safe.
It is important to improve our knowledge of contaminants present and their potential to cause harm in order to make appropriate risk-based assessments.
1EFSA, scientific opinion on the criteria to be used for safety evaluation of a mechanical recycling process to produce recycled PET intended for manufacture of materials and articles in contact with food, EFSA Journal 2011 ;9(7) :2184
2The assumption that the amount of contamination with PCR substances in the PET flakes is 3 mg/Kg is based on the EU project FAIR-CT98-4318. In this project, bottles collected from 12 European countries were examined. In individual cases, bottles were found that had been used for different purposes (i.e. not for food, but for storing chemicals). These bottles showed contamination with toluene and xylene up to a maximum of 6750 mg/Kg. A total of 3 such bottles were found, in the total collection of between 7000 and 10,000 bottles. This leads to an incidence of 0.03-0.04%. Based on the maximum found contamination value of 6750 mg/Kg, the above incidence results in a contamination of 2.7 mg/Kg, which the EFSA rounds up to 3 mg/Kg.
3EFSA, scientific opinion of the process Suplet Plasticos, EFSA Journal 2021 ;19(10).6867
4EFSA, Safety Assessment of the process POLY RECYCING ET DIRECT IV+…, EFSA Journal 2019 ;17(10) :5865
5EFSA, Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment, EFSA Journal 2019 ;17(6) :5708
6Mineral water example: with an EFSA-assumed body weight for small children of 5 kg, the consumption quantity of mineral water is set at 0.75 liters per day. It is further assumed that the water comes from 100% recycled PET bottles that have been in storage for 365 days at 25°C. This means that a PCR substance (or a surrogate) must not migrate in quantities higher than 0.017 µg/Kg in order not to be a danger to small children. This value is calculated from the maximum permissible intake limit for potentially genotoxic substances of 0.0025 µg per Kg of body weight per day. With a weight of 5 kg, a small child is permitted to ingest five times this amount, which results in 0.17 µg per day.
7Franz, R. ; Welle,F., Recycling of Post-Consumer Packaging Materials into New Food Packaging Applications-Critical Review of the European Approach and Future Perspectives. Sustainability 2022, 14, 824.
8Here the proposal is that the assumed genotoxic contamination level is set at 0.5 mg/Kg. This would reflect the empirical data, and take into account the uncertainty regarding the presence of genotoxic substances below the detection limit.
9Franz, R. ; Welle,F., Contamination Levels in Recollected PET Bottles from Non-Food Applications and their Impact on the Safety of Recycled PET for Food Contact. Molecules, 2020, 25, 4998.
